好ましくないジャーナリズム用語として「狂牛病」(Bovine Spongiform encephalopathy, BSE)
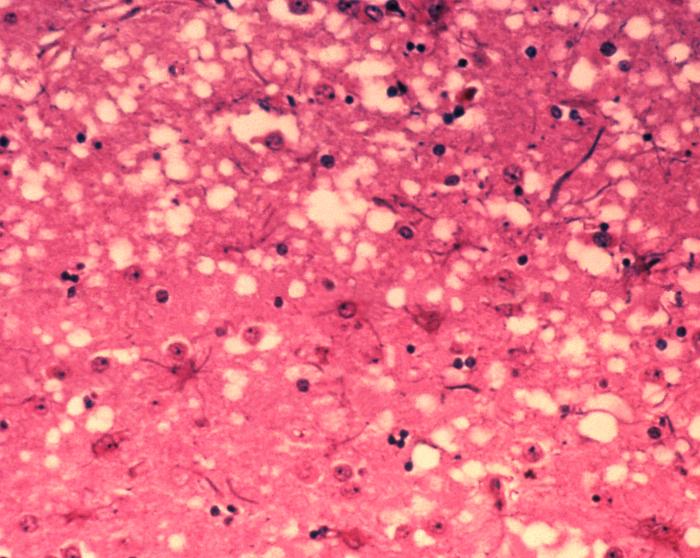
Brain tissue of a cow with BSE showing the typical microscopic "holes" in the grey matter - Bovine spongiform encephalopathy (BSE).
牛海綿状脳症
好ましくないジャーナリズム用語として「狂牛病」(Bovine Spongiform encephalopathy, BSE)

Brain tissue of a cow with BSE showing the typical microscopic "holes" in the grey matter - Bovine spongiform encephalopathy (BSE).
解説:池田光穂
異常型プリオンという熱につよいタンパク質によって伝染する中枢神経性の病気。牛海綿状脳症という(狂牛病は、牛の名誉=牛権のために好ましく ないので文化人類学者はあまり使わないほうがいい)
"Bovine spongiform encephalopathy
(BSE), commonly known as mad cow
disease, is an incurable and invariably fatal neurodegenerative
disease of cattle.[2] Symptoms include abnormal behavior, trouble
walking, and weight loss.[1] Later in the course of the disease the cow
becomes unable to function normally.[1] The time between infection and
onset of symptoms is generally four to five years.[2] Time from onset
of symptoms to death is generally weeks to months.[2] Spread to humans
is believed to result in variant Creutzfeldt–Jakob
disease (vCJD).[3] As of 2018, a total of 231 cases of vCJD had
been reported globally.[5]
BSE is thought to be due to an infection by a misfolded protein, known
as a prion.[3][6] Cattle are believed to have been infected by being
fed meat-and-bone meal (MBM) that contained either the remains of
cattle who spontaneously developed the disease or scrapie-infected
sheep products.[3][7] The outbreak increased throughout the United
Kingdom due to the practice of feeding meat-and-bone meal to young
calves of dairy cows.[3][8] Cases are suspected based on symptoms and
confirmed by examination of the brain.[1] Cases are classified as
classic or atypical, with the latter divided into H- and L types.[1] It
is a type of transmissible spongiform encephalopathy (TSE).[9]
Efforts to prevent the disease in the UK include not allowing any
animal older than 30 months to enter either the human food or animal
feed supply.[4] In continental Europe, cattle over 30 months must be
tested if they are intended for human food.[4] In North America, tissue
of concern, known as specified risk material, may not be added to
animal feed or pet food.[10] About four million cows were killed during
the eradication programme in the UK.[11]
Four cases were reported globally in 2017, and the condition is
considered to be nearly eradicated.[1] In the United Kingdom, from 1986
to 2015, more than 184,000 cattle were diagnosed with the peak of new
cases occurring in 1993.[3] A few thousand additional cases have been
reported in other regions of the world.[1] It is believed that several
million cattle with the condition likely entered the food supply during
the outbreak.[1]"- Bovine
spongiform encephalopathy (BSE).
異常型プリオンに感染すると、体内にある正常なタイプのプリオンが変化すると考えられている。細菌やウイルスのように、遺伝情報が複製されて増 殖するというタイプの病気とはことなるために、このような発症のメカニズムが長くわからなかった。
脳の抽出物(硬膜や脳髄など)の直接の移植や、経口からからの摂取によって引き起こされる。
人間では、クロイツフェルト−ヤコブ病や、ニューギニアで報告されたクールーという病気に類似の症状がでるので、おなじ病気だといわれている (プリオンは哺乳類を超えて感染力があると考えられている)。
牛は、本来草食性であるので、そのような病気に感染するはずがないのだが、人間に対する食肉需要から、人間が近年になって、高タンパク質つま り、屠畜され食肉として利用後に残った骨や内臓あるいは脳などの動物飼料——海綿状脳症についての症例はヤギでは早くから報告されていた——を牛に使うよ うになってから、その流行の規模が拡大したと指摘されている。
Pathogenesis, "The pathogenesis of BSE is not well understood or documented like other diseases of this nature. Even though BSE is a disease that results in neurological defects, its pathogenesis occurs in areas that reside outside of the nervous system.[20] There was a strong deposition of PrPSc initially located in the ileal Peyer's patches of the small intestine.[21] The lymphatic system has been identified in the pathogenesis of scrapie. It has not, however, been determined to be an essential part of the pathogenesis of BSE. The Ileal Peyer's patches have been the only organ from this system that has been found to play a major role in the pathogenesis.[20] Infectivity of the Ileal Peyer's patches has been observed as early as 4 months after inoculation.[21] PrPSc accumulation was found to occur mostly in tangible body macrophages of the Ileal Peyer's patches. Tangible body macrophages involved in PrPSc clearance are thought to play a role in PrPSc accumulation in the Peyer's patches. Accumulation of PrPSc was also found in follicular dendritic cells; however, it was of a lesser degree.[22] Six months after inoculation, there was no infectivity in any tissues, only that of the ileum. This led researchers to believe that the disease agent replicates here. In naturally confirmed cases, there have been no reports of infectivity in the Ileal Peyer's patches. Generally, in clinical experiments, high doses of the disease are administered. In natural cases, it was hypothesized that low doses of the agent were present, and therefore, infectivity could not be observed.[23]"- Bovine spongiform encephalopathy (BSE).
●狂牛病と牛海面状脳症とはどうちがうのか?
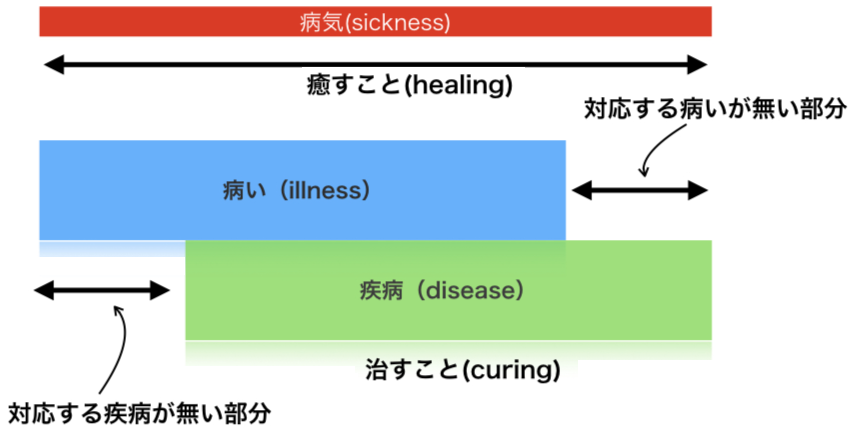
端的に言うと、狂牛病は「病い(illness)」であり、牛海面状脳症(BSE)は疾病(しっぺい)である。ふつうの人たちが理解し、感じて いる病気の概念や経験を「病い・やまい」(illness)とよび、医療の専門家—— とくに医師ないしは彼/彼女らが依拠する生物医学(biomedicine)——が定義する病者への診断のことを「疾病・しっぺい」(disease)と 呼ぶ(→詳しくは「病いと疾病」に)。
リンク
【文献】

Copyleft, CC, Mitzub'ixi Quq Chi'j, 1996-2099